You may have heard that without distillation, there would be no spirits - this is true! You may also have heard that the distillation process is really complicated and hard to understand - this is less true. Like any subject, distillation can be explored with varying degrees of complexity, but we like to start with the basics. Let's simplify the spirits distillation process down to a few underlying ideas that anyone can understand.
Firstly, what does distillation do?
A distiller starts with a raw material - it could be grains, grapes, agave, sugar cane - anything of agricultural origin. The first step in spirits production is to make a sugary liquid from this raw material.
Then, the distiller adds yeast to the sugary liquid. Yeast are microorganisms that eat sugar and make the alcohol ethanol. Once they have eaten all the sugars, typically about 8-10% of the volume of the liquid is ethanol. Most of the remaining liquid is water, but it also includes many other components. Altogether the components that make up a liquid are called 'fractions'.
The fractions other than ethanol and water only make up a small percentage of the liquid volume, but there are still hundreds if not thousand of these other fractions. They either came from the raw material or were created by the yeast.
If you're looking to begin your journey into spirits education, the WSET Level 1 Award in Spirits would be a great fit for you. Click here to discover more about the course.

Harvesting raw materials - sugar cane in this image
Through the technique of distillation, some of these fractions can be selected and concentrated. For example, through distillation the percentage of ethanol in a liquid can increase from just 8-10% to 60% (and in most cases much more).
Despite their alcoholic strength, spirits are not about alcohol - they're about flavour. The true aim and art of distillation is to select and concentrate the desired delicious flavours in the fermented liquid.
How does distillation select and concentrate fractions?
It all comes down to boiling points. While pure water boils at 100°C (212°F), pure ethanol boils at 78.3°C (173°F) — this means it takes less energy for ethanol to become a gas.
You can see this process for yourself when you boil a pan of water on the stove. The bubbles that appear are just water in the form of a gas being forced out of the liquid.
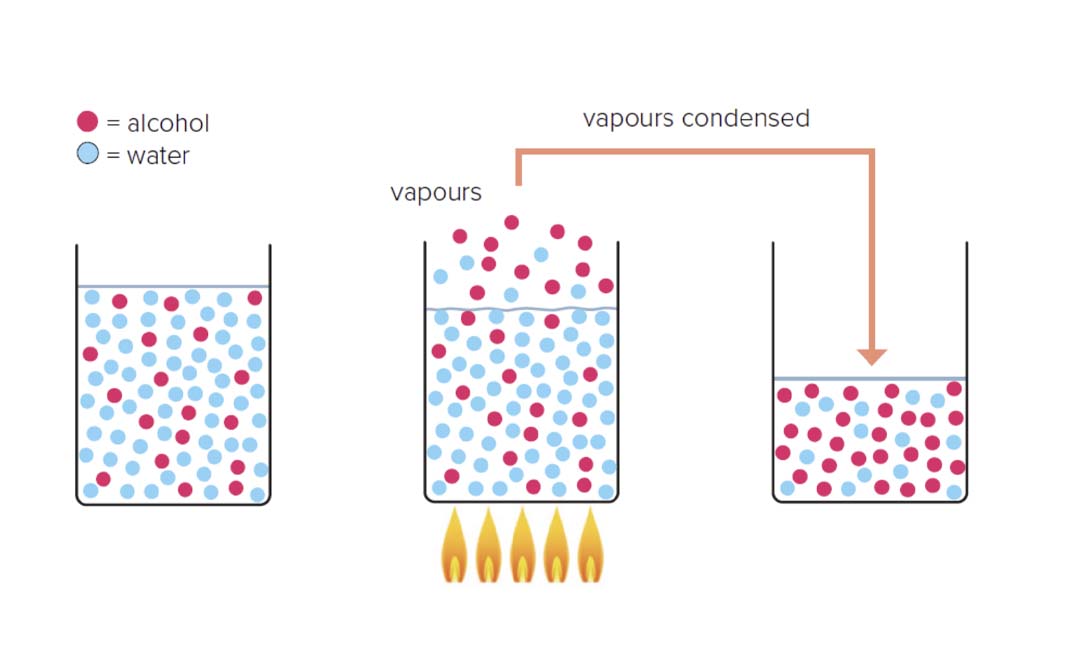
Let's move on now and imagine a liquid made up of just two fractions, 10% ethanol and 90% water. When this is heated up, it boils, and gas bubbles out of it.
As it takes less energy for ethanol to boil than water, that gas will be about 50% ethanol and just 50% water. So in this simplified example, the ethanol has been selected and concentrated, as the gas that has "escaped" from our boiling liquid will contain more ethanol.
We know that in the imaginary liquid from our example, the gas will have a higher concentration of the fraction with the lower boiling point, ethanol.
The same principle applies if there are hundreds of fractions (as there are in a fermented alcoholic liquid used in spirits production) rather than just two. The fraction with the lowest boiling point will be most concentrated first.
Distillation takes place in a 'still', and no matter how complex or fancy a 'still' it is, you rely on this simple principle of selection and concentration in order to create the spirits that we enjoy.
With thanks to Nick King DipWSET, WSET's Head of Product Development (Spirits), for putting this post together.
Related content: